The Strange and Squishy World of Non-Newtonian Fluids
Why Slime Isn’t Just for Kids
Imagine this: you open a bottle of ketchup, give it a polite shake, and nothing happens. So, you shake harder, and suddenly — a tsunami of ketchup floods your fries. Or perhaps you’ve played with slime or mixed cornstarch and water at home, only to discover that it feels solid when you punch it, but flows like liquid when left alone.
These everyday curiosities may seem trivial or even annoying, but they’re windows into a fascinating and surprisingly complex corner of physics — the world of non-Newtonian fluids.
In this post, we’ll explore what makes these fluids so unusual, what’s going on at the microscopic level, and why understanding them isn’t just fun — it’s also scientifically and technologically important.

What Is a Fluid, Anyway?
To appreciate non-Newtonian fluids, we should first define what we mean by a fluid. In physics, a fluid is any substance that can flow — this includes both liquids and gases. Fluids take the shape of their container, can be poured, and generally behave in predictable ways… unless they don’t.
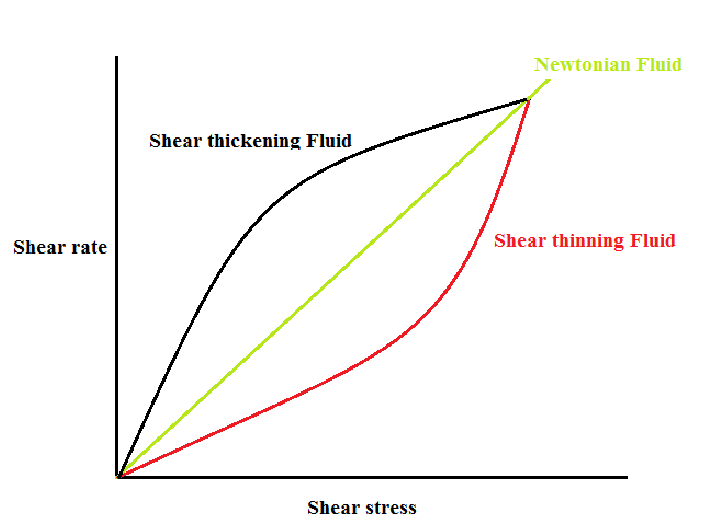
Most of the fluids we encounter — water, juice, air — are what physicists call Newtonian fluids, named after Sir Isaac Newton. These obey a very straightforward rule: their viscosity (a measure of a fluid’s resistance to flow, or in simpler terms, its “thickness”) remains constant regardless of how much force you apply.
If you stir water slowly or rapidly, its viscosity doesn’t change. It remains water — consistent, reliable, and obedient to Newton’s laws of motion.

When Fluids Misbehave: Non-Newtonian Fluids
Non-Newtonian fluids, on the other hand, are the rebels of the fluid world. Their viscosity changes depending on the kind of stress you apply — whether it’s squeezing, stirring, pressing, or shaking.
This strange behaviour can manifest in a few different ways. The two most common are:
- Shear thinning: The fluid becomes less viscous (thinner) under stress.
- Shear thickening: The fluid becomes more viscous (thicker) under stress.
Let’s look at some real-world examples.
Slime: A Playful Polymer Puzzle
Slime is perhaps the most hands-on introduction to non-Newtonian behavior. When you pull it slowly, it stretches. But yank it quickly, and it snaps or tears. Press it gently and it oozes; press hard, and it stiffens.
What’s happening here is a dance of polymer chains. Slime is made of long, flexible molecules — like cooked spaghetti — tangled up with each other. When you move slowly, these chains have time to slide past one another. But if you act quickly, the chains can’t reorganize fast enough and they temporarily lock in place, giving the slime a solid-like feel.
This isn’t just a party trick — it’s a glimpse into viscoelasticity, where materials exhibit both fluid and solid characteristics depending on the situation.

The Ketchup Conundrum: Shear Thinning in Action
Ketchup is another classic non-Newtonian fluid — and one that many people battle with during dinner.
At rest, ketchup sits stubbornly in the bottle, thick and unmoving. But apply a sudden squeeze or shake, and it flows rapidly. This is shear thinning: under increased shear force (in this case, your squeezing or shaking), the ketchup’s internal structure breaks down, and it becomes less viscous.
Why does this matter? Apart from saving your fries from over-saturation, shear thinning is crucial in industries like paint manufacturing, where a substance needs to spread easily under a brush but stay in place once applied.

Cornstarch and Water: The Oobleck Phenomenon
Now for something even weirder: a mixture of cornstarch and water, sometimes affectionately called Oobleck, after a substance from a Dr. Seuss book.
If you punch a bowl of Oobleck, it feels like a solid. But rest your hand gently on it, and it melts around your fingers. This is shear thickening: the more force you apply, the thicker and more solid the mixture becomes.
This counterintuitive behavior arises because the cornstarch particles are suspended in water. When left alone or moved slowly, the particles have space to flow past each other. But when you apply sudden force, the particles are pushed together, forming a temporary solid-like network.
Researchers are exploring shear-thickening fluids like this for protective applications — such as liquid body armor that remains flexible during movement but hardens instantly upon impact.

What’s Going On at the Microscopic Level?
At the heart of non-Newtonian behaviour is the internal structure of the material.
In Newtonian fluids, molecules move freely and independently. But in non-Newtonian fluids, there’s structure — particles, polymers, or microscopic interactions that respond to stress. These structures can align, compress, disentangle, or form temporary networks, altering how the material flows.
In simple terms, these fluids “feel” the way you treat them and adjust their behaviour accordingly. It's like dealing with a moody friend — gentle handling gets one response, abrupt pressure gets another.
More Than Just Kitchen Curiosities
Non-Newtonian fluids are not rare or obscure — they’re all around us.
- Blood is a non-Newtonian fluid. Its viscosity decreases with increasing flow rate, helping it move more efficiently through our circulatory system.
- Cosmetic creams and lotions are engineered to shear thin for smooth application but retain thickness on the skin.
- Quicksand behaves like a non-Newtonian fluid — struggle, and it stiffens; stay still, and you may slowly sink.
- Drilling mud used in oil wells changes viscosity depending on pressure to prevent blowouts.
- 3D printing materials often rely on shear-thinning behaviour for precise extrusion.
In engineering, medicine, food science, and materials design, understanding how fluids behave under stress helps us create better products — and sometimes even save lives.

In Conclusion: The Science Beneath the Goo
The world of non-Newtonian fluids is strange, surprising, and deeply useful. These materials challenge our intuitions and remind us that not all scientific principles are as tidy as we might hope. Some things stiffen when shaken; others melt when touched. And all of them reflect the rich complexity of the microscopic world.
So the next time you're fighting with ketchup, poking at slime, or experimenting with cornstarch in your kitchen, take a moment to appreciate the physics in your hands.
You’re not just making a mess.
You’re exploring a boundary where matter behaves unpredictably — and where science, fun, and everyday life converge.
Try This at Home:
Want a safe and fascinating experiment?
Mix about 1 cup of cornstarch with ½ cup of water. Stir slowly until combined. Then try tapping it, punching it, or letting your hand sink into it.
Just don’t do it near your carpet.

Member discussion